UCT’s exclusive testing technology detects and quantitates the CA-62 biomarker specifically for epithelial carcinomas wherever they are in the body. During the malignant transformation of various epithelial cells, they transform into pluripotent stem cells. The membrane-type glycoprotein CA-62 appears uniquely on the surface of these cancer cells. This biomarker enters the intracellular space and then circulates in the bloodstream where it can be detected by UCT’s CA-62 Biomarker Cancer Test. Quantitation allows for the determination of the likelihood of the presence of cancer in a patient with high sensitivity and specificity. Levels of CA-62 are highest during the early stages of cancer making it an exceptional tool for reliable early detection for a range of epithelial cancer types.
Earlier detection can mean more effective treatments and higher survivability for patients around the globe.
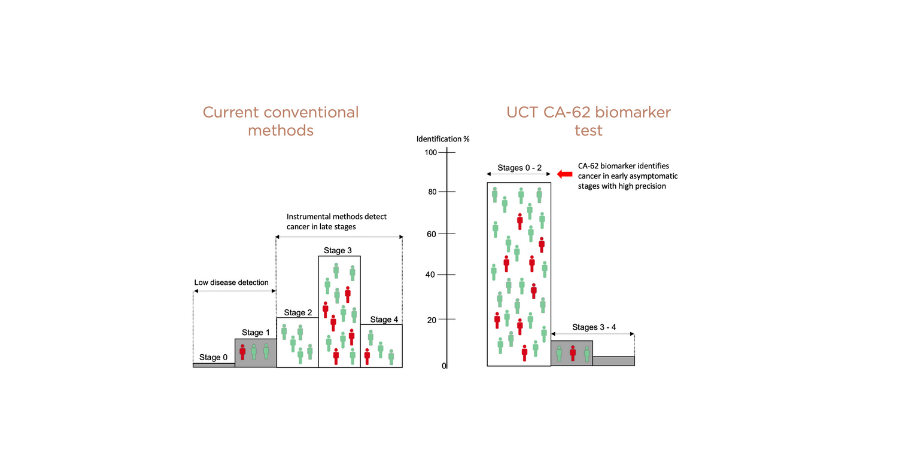
The majority of cancers are being detected at advanced stages, when the clinical prognosis is unfavorable and results in high mortality rates. This is primarily because cancer does not typically become symptomatic until these later stages. Screening for cancer within a generally-healthy population using the highly sensitive UCT CA-62 Biomarker Cancer Test effectively detects a range of cancers from Stage I onward.
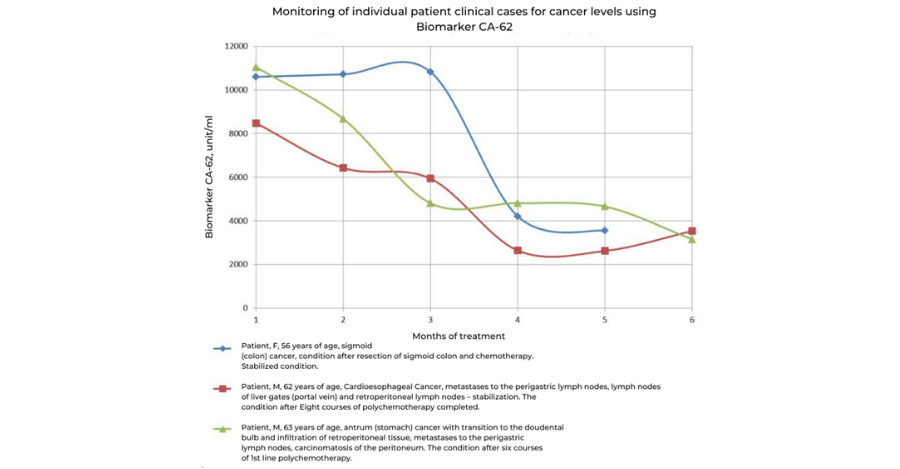
Patients with disseminated cancers (carcinomas) can be monitored as a growth inhibition indicator using the UCT CA-62 Biomarker Cancer Test. The rapid results may be useful in the assessment of the ongoing treatment success and for the timely detection of cancer chemotherapy resistance. This allows oncologists to make timely decisions regarding the modification or replacement of prescribed chemotherapy treatments based on realtime results. At present there are no other highly sensitive biomarkers that can reflect the tumor response to an ongoing cancer therapy. UCT’s innovative testing technology can also be used in a clinical practice to monitor malignant neoplasms of the gastrointestinal tract, ovaries, lungs, large intestine, and rectum.
Early detection of cancer recurrence is now possible using the UCT CA-62 Biomarker Cancer Test. The dynamics of the CA-62 biomarker can serve as a significant prognostic factor for the early detection of cancer recurrence. A steady or sharp increase in biomarker’s level during the remission is likely to be related to continued tumor growth and disease progression, which can be confirmed using conventional instrumental methods such as NMR, CT-scan, an ultrasound etc.
UCT has been developing its innovative UCT CA-62 Biomarker Cancer Test since it was first conceived in 2012. Since that time UCT has tested more than 5,000 human blood samples , with very positive results. The tests were conducted in a number of international centres including Canada, United States, the Russian Federation, and Kazakhstan across a range of different epithelial cancer types including breast cancer, prostate cancer, stomach cancer, colorectal cancer, lung cancer, liver cancer, cervical cancer, ovarian cancer, melanoma, intestinal cancer, myeloma, esophageal cancer, glioblastoma.
The results of these studies clearly showed that UCT’s CA-62 Biomarker Cancer Test was able to detect cancer from these samples with >90% sensitivity and 95% specificity. The data generated supported the approval of the medical device filings in the Russian Federation and Republic of Kazakhstan bringing this powerful testing technology to patients in its first two markets.
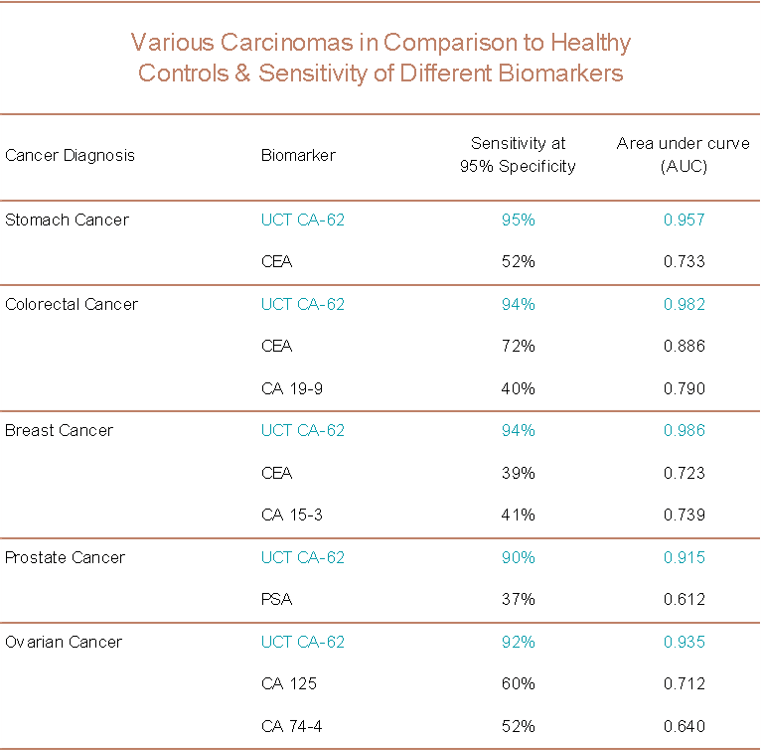
The table summarizes the sensitivity of the UCT’s CA-62 Biomarker Cancer Test as compared to other available biomarkers for specific epithelial cancer types.
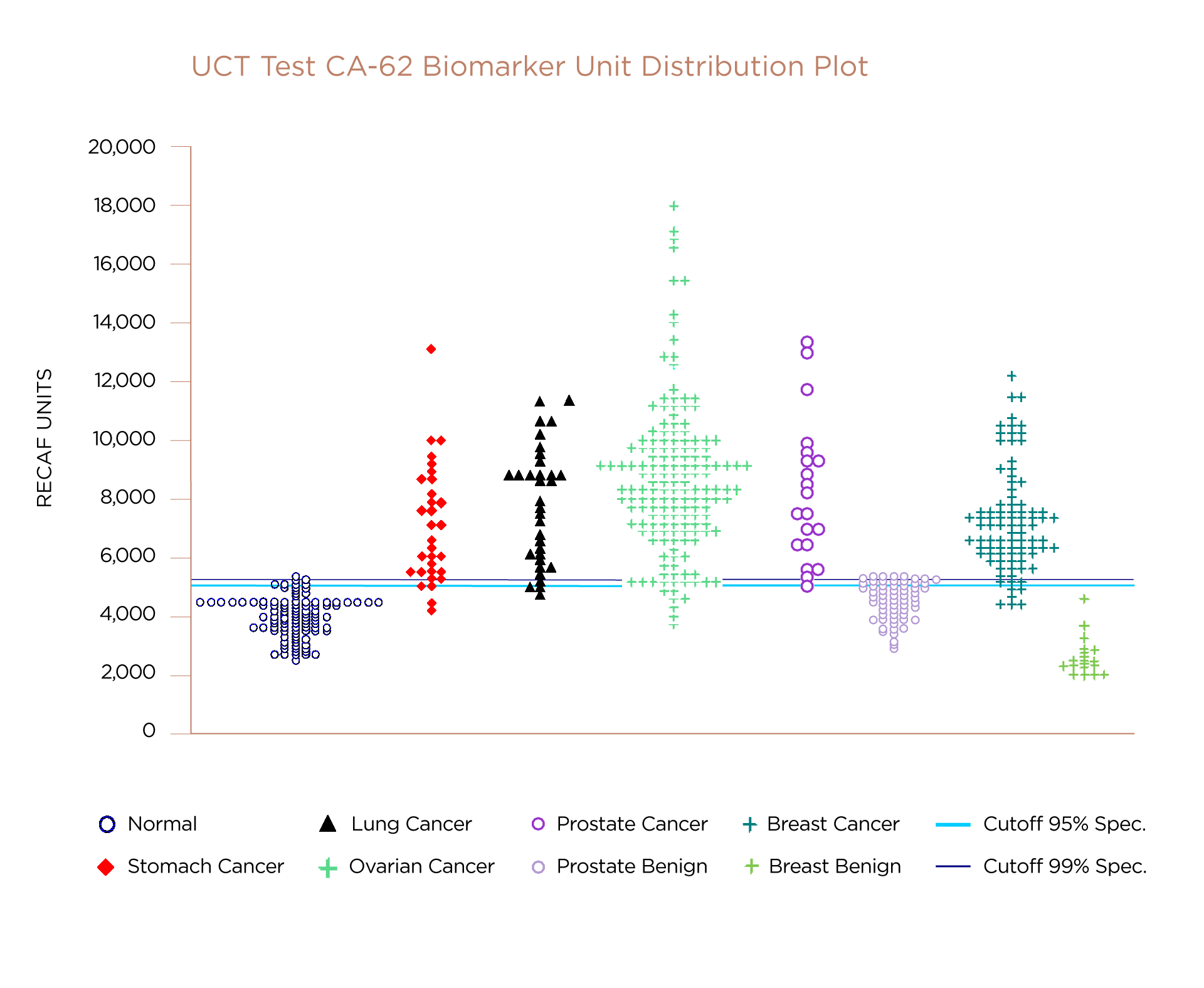
The UCT CA-62 Biomarker Cancer Test has been challenged against a number of different epithelial cancer types. This graph marks the level of CA-62 biomarker (RECAF units) measured in samples from studies. The horizontal line indicates the minimum level of CA-62 biomaker that indicates the likely precense of cancer in the patient. Note that the results for independently confirmed benign cases show a distribution of results all below this line indicating the absence of cancer. The data plotted above the line shows the distribution of CA-62 biomarker levels measured in subjects confirmed with a form of cancer.
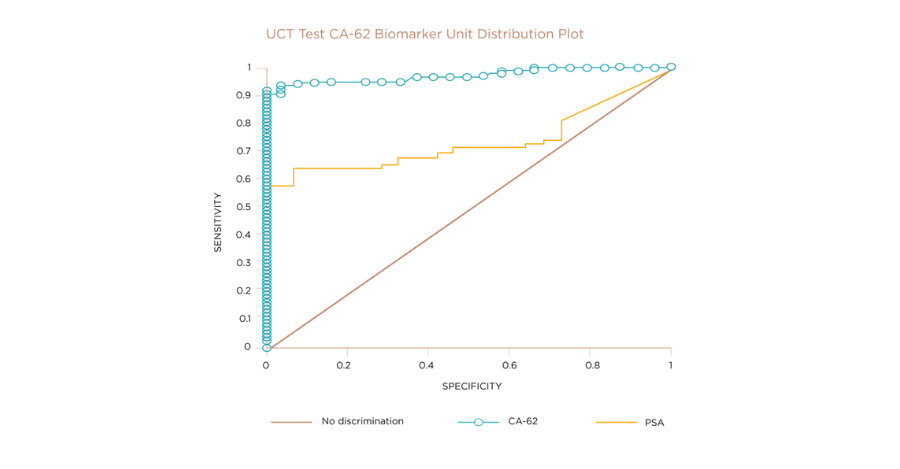
One type of cancer that can impact a large proportion of the male population is prostate cancer. Currently the main test deployed to detect prostate cancer is the PSA marker. The graph compares the sensitivity and specificity of the PSA and UCT CA-62 biomarker tests, on approximately 2,000 subject samples. While the conventional PSA marker test showed sensitivity of approximately 60% , UCT’s CA-62 Biomarker Cancer Test provided a sensitivity of over 90% and returned much fewer false positives. When UCT’s CA-62 Biomarker Cancer Test has been used for prostate cancer detection, it has demonstrated superior sensitivity and specificity to the conventional PSA test.
Scientific Publications
- CA-62: A new biomarker for the detection of early-stage renal cell carcinoma. Janneta Tcherkassova, Sergei Tsurkan, Anna Prostyakova, Evgueni Klinski, Marina Sekacheva, Alexander Boroda, and Ricardo Moro. Journal of Clinical Oncology Volume 42, Number 16_suppl
- A novel three-biomarker signature (CA-62, CEA, CYFRA 21-1) and early-stage NSC lung cancer detection. Journal of Clinical Oncology Volume 41, Number 16_suppl June 2023
- POTENTIAL CLINICAL APPLICATION OF THE CANCER ANTIGEN CA-62 FOR DIFFERENTIAL DIAGNOSIS OF PROSTATE CANCER AND BENIGN PROSTATIC HYPERPLASIA AT THE ELEVATED PROSTATE-SPECIFIC ANTIGEN Zh. R. Cherkasova, S. A. Tsurkan, A. I. Prostyakova, A. M. Boroda, A. A. Rozhkov, Yu. N. Pirogova, Research and Practical Medicine Journal. 2023. Vol. 10, No. 1. P. 10-26
- Diagnostic efficacy of the new prospective biomarker’s combination CA 15-3 and CA-62 for early-stage breast cancer detection: Results of the blind prospective-retrospective clinical study. Cancer Biomarkers 35 (2022) 57–69
- Clinical validation of the novel CLIA-CA-62 assay efficacy for early-stage breast cancer detection Frontiers in Oncology, 02 May 2023 Sec. Breast Cancer, Volume 13 - 2023
- J. Cherkassova, A. Prostyakova, S. Tsurkan, V. Ragoulin, A. Boroda, and M. Sekacheva. Diagnostic Efficacy of the new prospective biomarker's combination CA 15-3 and CA-62 for early-stage breast cancer detection: Results of the blind prospective-retrospective clinical study. Cancer Biomarkers, vol. Pre-Press, no. Pre-Press, pp. 1-13, 2022
- A pilot clinical trial to monitor response to chemotherapy using the CA-62 marker of epithelial carcinomas. Khakimova Gulnoz G., Cherkasova Zhanneta R., Tsurkan Sergey A., Fedchikov Gleb A., Suganov Nikolay V., Gorbunova Vera A. Siberian Journal of Oncology, 2019, V.18, N.5, p. 18-28.
- Tcherkassova J.R., Tsurkan S.A., Smirnova G.B., Borisova J.Y., R. Moro, and Treshalina H.M. Binding characterization of the targeting drug AIMPILA to AFP receptors in human tumor xenografts. Tumor Biology, 2017, Oct, 9, p. 1-10.
- Tsurkan S., Tcherkassova J., Gorbunova V., Treshalina H. New drug AIMPILAa targeted to AFP receptor: oral anticancer therapy and biodistribution in vivo. Journal of Clinical Oncology, 2018, Т. 15_suppl. С. e24232.
- Treshalina H.M., Smirnova G.B., Tsurkan S.A., Tcherkassova J.R., Lesnaya N.A. The role of alpha-fetoprotein receptor in the delivery of targeted preparations in oncology. Russian Journal of Oncology, 2017, Vol. 22, N 1, p.4-14 ISSN 2412-9119.
- Cherkasova Zh.R., Tsurkan S.A., Smirnova G.B., Borisova Yu.A., Treshalina E.M. Expression of AFP-receptors in human tumor homogenates SW620, T47D and in cell culture HepG2 from the collection of the Blokhin Russian Cancer Research Centre. Russian Journal of Biotherapy, 2016, V. 1, N.15:p.116-7.
- Cherkasova Zh.R., Tsurkan S.A., Smirnova G.B., Borisova Yu.A., Treshalina E.M. Binding specificity of Aimpila with AFP receptors on the surface of human tumor T47D from the collection of the Blokhin Russian Cancer Research Centre. Russian Journal of Biotherapy, 2016, V.1, N.15, p.117.
- Ricardo Moro, Janneta Gulyaeva-Tcherkassova, Petra Stieber. Increased AFP-Receptor (RECAF) values in the serum of patients with early stages of breast cancer. Journal of Current Oncology, 2012, Vol. 19, N.1, p. 1-8.
- Janneta Tcherkassova, Carolina Abramovich, Rafael Moro, Chen Chen, Ralph Smit, Angela Gerber, Ricardo Moro. Combination of CA125 and RECAF biomarkers for early detection of ovarian cancer. Tumor Biology, 2011, Vol. 32, No.4, p.831-838.
- Irene NG, Janneta Tcherkassova, Nina Lyubimova, Ricardo Moro. A new RECAF ELISA and Its Correlation with the Chemiluminescence RECAF Assay. Tumor Biology, 2008, V. 29 (Suppl. 1), p. 41.
- Barry Dowell, Stephen Frost, Janneta Tcherkassova, Angela Gerber, Rafael Moro, and Ricardo Moro. Chemiluminescent assay (CIA) for the receptor of ALPHA FETOPROTEIN (RECAF) to separate cancer from normal sera. Tumor Biology, 2007, V. 28 (Suppl. 1), p.92
- Janneta Tcherkassova, Ralph Schmid, Xiaolong Hu, Nina Lyubimova, Ricardo Moro. Point-of-care serum test for cancer detection based on the RECAF cancer marker. Tumor Biology, 2007, V. 28 (Suppl. 1), p.101
- Ricardo Moro, Angela Gerber and Janneta Tcherkassova. High Discrimination between Prostate Cancer, Benign and Normal Serum Samples Using RECAF. Tumor Biology, 2006, V. 27 (Suppl. 2), p.57
- R. Moro, J. Tcherkassova, A. Gerber. High discrimination between Prostate cancer, Benign and Normal serum samples using the cancer marker RECAF. Journal of Clinical Chemistry, 2005, V. 52, Suppl. 6, p. 26
- https://ucttest.com/wp-content/uploads/2020/12/Ricardo2016NovaScience-122648.pdf
- Moro R., Tcherkassova J., Smith R., Gerber A., Moro R. RECAF marker reduce unnecessary prostate biopsies by 70% while detecting 80% of prostate cancer at stages I & II and 92% at all stages. 2021, in press.
- Moro R. Combination of RECAF with other markers improves cancer diagnosis. Abstracts of ISOBM 2017 Congress for Tumor Marker Publication, Tumor Biology, December 2017, p. 16.
- Moro R. The alpha-fetoprotein receptor (RECAF): characterization and potential use for cancer diagnosis and therapy. Alpha-Fetoprotein: Functions and Clinical Applications Chapters Books, Nova Science Publishers, 2016, p.241-276
- Moro R., Gulyaeva–Tcherkassova J., Stieber P. Increased alpha-fetoprotein receptor in the serum of patients with early-stage breast cancer. Current Oncology, 2012 Vol. 19 (1), p. 1-8
- Tcherkassova J, Moro R. RECAF as a replacement for free PSA in prostate cancer detection. Tumor Biology 2011, V 32, p.71
- Tcherkassova J, Abramovich C, Moro R, Chen Chen, Schmit R, Gerber A, Moro R. Combination of CA125 and RECAF biomarkers for early detection of ovarian cancer Tumor Biology, 2011, Vol. 32, No.4, p. 831-838
- Moro R, Tcherkassova J, and Moro R.J. Combination of CEA and the receptor to AFP (RECAF) for colorectal cancer screening. IOBM meeting, 2009, Amsterdam, Holland.
- B.Dowell,S.Frost (Abbott laboratories), J.Cherkassova, G.Gerber, R.Moro and R.Moro . Development of a Chemiluminescent Assay for the Receptor of Alha Fetoprotein to Separate Cancer from Normal Sera. XXXVth Congress of the International Society of Oncodevelopment Biology and Medicine, 2007, Prague, Czech Republic.
